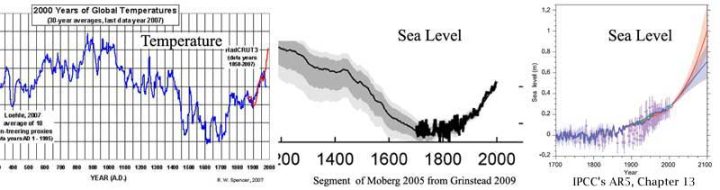
by K. Richard, Oct 24, 2022 in NoTricksZone
The Earth was still in ice age conditions 14,700 to 12,900 years ago, or during the “Bolling interstadial.” CO2 hovered near 230 ppm at that time, and yet “continental Europe was a few degrees warmer than present” (Toth et al., 2022).
In recent years there have been multiple studies detailing a European climate that was as warm or warmer than today during the late Pleistocene ice age.
The latest study, Toth et al., 2022, uses chironomid proxy evidence to reconstruct summer temperatures at a lake site in the Eastern Carpathians.
These authors report that “continental Europe was a few degrees warmer than present during the Bolling interstadial,” and there were slightly (0.5°C) warmer-than-today periods (e.g., ~16,300 years ago) at the study site. The warming events were both pronounced (5°C) and abrupt.
…